Describe the Process of Diffusion Using the Particle Theory
Diffusion is a process of spreading a substance from a region of high concentration to a region of low concentration. Using particle theory imagine that you have a substance ie perfume.
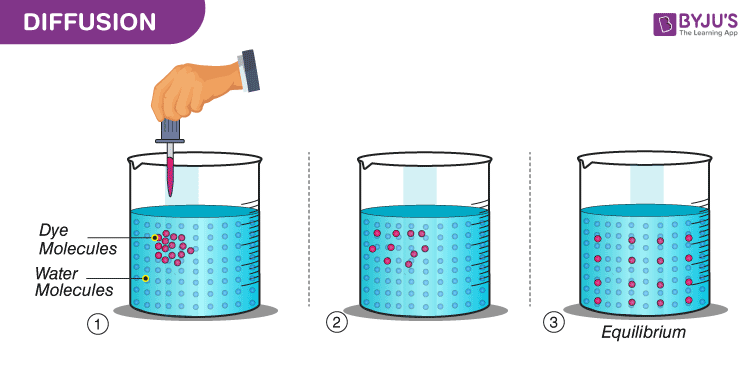
What Is Diffusion Definition Types Examples Of Diffusion
Osmosis is the process of.

. Your understanding of using models can be enhanced with the RSC CPD course Developing and using models. The particles move in order to create equal concentration from higher to lower between all spaces of a place. The material that diffuses could be a solid liquid or gas.
Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower concentration. Thus diffusion should not be confused with convection or dispersion which are other transport. The central idea of diffusion however is common to all of these.
Diffusion is driven by differences in concentration. A scientific model is a way of illustrating ideas objects and processes so theyre easier to understand. When chemical substances such as perfume are let loose in a room their particles mix with the particles of air.
Boiling point is the temperature at which the vapour pressure of a liquid equals the external. The particles of smelly gas. 7 D U l 2 2 d τ.
That all particles are in constant motion. Similarly the medium in which diffusion occurs could also be in one of the three physical states. Diffusion of particles provides many useful contexts to help students make the link between the macroscopic world and the sub-microscopic.
Up to 24 cash back sugar particle colour particle water flavour particle This picture shows the mixed particles of a fruit-flavoured drink. A substance or collection undergoing diffusion spreads out from a point or location at which there is a higher. Diffusion increases entropy randomness decreasing Gibbs free energy and therefore is a clear example of thermodynamicsDiffusion operates within the boundaries of the Second Law of Thermodynamics because it demonstrates natures tendency to wind down to seek a state of less concentrated energy as evidenced by increasing entropy.
In probability theory and statistics a diffusion process is a solution to a stochastic differential equationIt is a continuous-time Markov process with almost surely continuous sample paths. A sample path of a diffusion process models the trajectory of a particle. Molecules move around by diffusion When two molecules come close together they have some probability of reacting to combine or modify one another Two implementation.
Diffusion is the movement of particles from a high concentration to a low concentration. Brownian motion is the random movement of microscopic particles suspended in a liquid or gas caused by collisions between these particles and the particles of the liquid or gas. The relation between the two diffusion coefficients.
Although the expansion of a solid is due to the higher average kinetic energy of the particles and the more energetic vibrations they are still held together by the intermolecular bonding forces or much stronger strong ionic or covalent bonds which restricts the expansion - this is not part of the kinetic particle theory. The Particle Theory of Matter is a scientific model. The rate of this movement is a function of temperature viscosity of the medium and the size mass of the particles.
Diffusion results in the gradual mixing of materials and eventually it forms a homogeneous mixture. Diffusion is almost impossible in solids because the particles are. It occurs when the particles of the substance move through the space between the particles of another substance.
Placing a drop of food coloring in water provides a visual representation of this process the color slowly spreads out through the water. Why is the volume of a sugar and water mixture less than the volume of each substance alone. An alternative paper-based activity is Particle model cards from the Royal Society of Chemistry.
Within the cell involves reaction-diffusion simulation Basic rules. This process is called diffusion. Diffusion is one of several transport processes that occur in nature.
For a diffusion process constituted of uncorrelated elementary jumps with average residence time τ ie jump rate τ1 and jump distance l the diffusion coefficient can be given as. Diffusion refers to the process of particles moving from an area of high concentration to one of low concentration. The concept of diffusion is widely used in many fields including physics particle diffusion chemistry biology sociology economics and finance diffusion of people ideas and price values.
Use the picture and the particle theory to explain what happens to the particles in this solution. A distinguishing feature of diffusion is that it results in mixing or mass transport without requiring bulk motion. Diffusion is the movement of particles from a high concentration to a lower concentration.
It can be seen as a spreading-out of particles resulting in their even distribution. The particle theory states that there are spaces between all particles. When two substances mix their particles intermingle until their composition is uniform throughout.
Diffusion is a process in which particles spread out through random movement from high to low concentration until they are evenly distributed. Diffusion follows a basic idea in the particle theory. Explore the relationships between ideas about the particle theory in the Concept Development Maps - States of Matter Atoms and Molecules Conservation of Matter Chemical Reactions Flow of Energy in Ecosystems Flow of Matter in Ecosystems Students will have a range of explanations for phenomena they have observed.
Basically since the particles are in constant motion they move. Brownian motion reflected Brownian motion and OrnsteinUhlenbeck processes are examples of diffusion processes. Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration.
Diffusion is the process by which particles of one substance spread out through the particles of another substance. This means that in a glass of water there are many water particles but also many empty spaces. Scientists use models to explain things we cant see without advanced equipment.

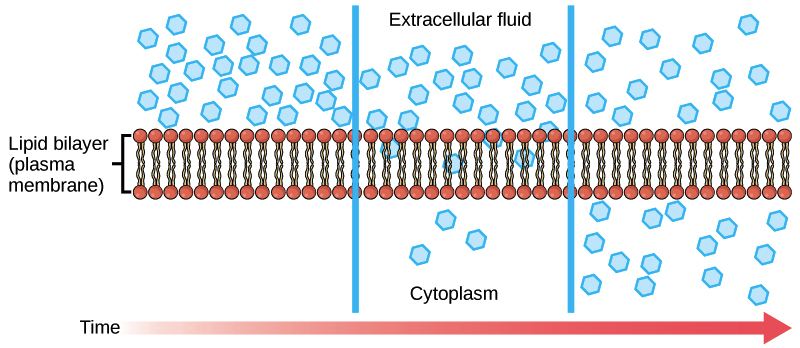
Diffusion And Osmosis Biology I Laboratory Manual
No comments for "Describe the Process of Diffusion Using the Particle Theory"
Post a Comment